
Crystallization is a process responsible for the formation of solid structures, wherein constituent atoms or molecules are systematically arranged into a highly organized lattice, constituting a crystal.
This phenomenon can occur through various mechanisms, including precipitation from a supersaturated solution, solidification from a liquid state, and, less commonly, direct sublimation from a gaseous phase. The characteristics of the resultant crystal are significantly influenced by a range of environmental conditions – temperature and atmospheric pressure.
Growth Process
The dendritic growth pattern in crystal formation provides a captivating example of fractal growth. The primary crystal structure, often initiated as a nucleus, undergoes recursive branching into smaller crystalline structures. Originally conceptualized as a dynamic process, crystal growth exemplifies a fundamental instance of self-similarity, demonstrating a mathematically generated pattern that preserves its intricate details at various scales of observation.
PseudoCode // Single Crystal
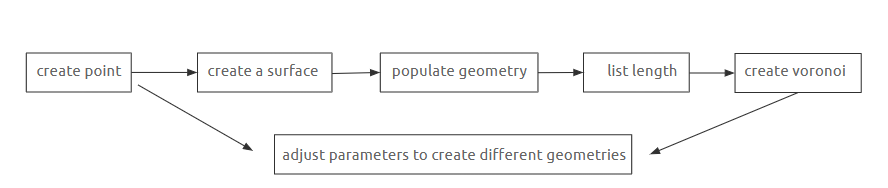
Crystal
GROWING CRYSTAL
Elements that define how the natural element is performing:
Fractal Dimension: A measure of how completely the fractal fills space, indicating the degree of complexity or roughness of the fractal structure. Fractal dimension quantifies the self-similarity of the pattern at different scales.
Branching Ratio: The ratio of the number of branches at each generation level in the fractal hierarchy. It characterizes the branching behavior of the crystal growth pattern.
Scaling Laws: Mathematical relationships that describe how certain properties of the fractal (e.g., length, area, volume) scale with changes in observation scale. Scaling laws provide insights into the underlying mechanisms driving fractal growth.
PseudoCode // Growing Crystal
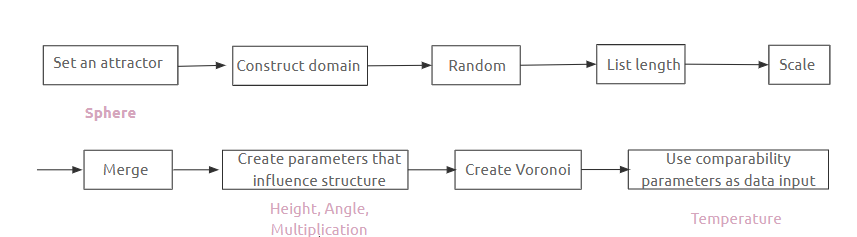
Growing Crystal
Explorations
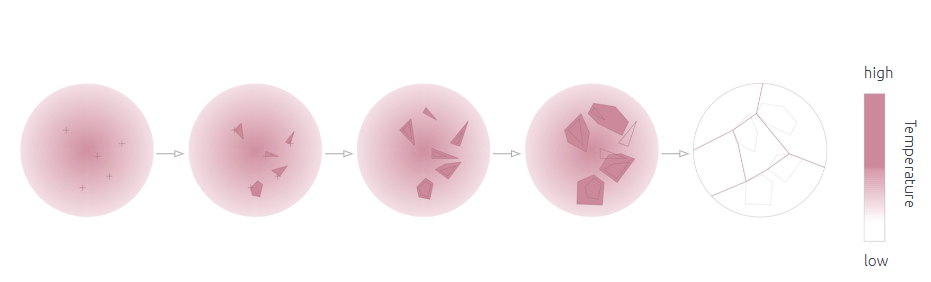
For the following exploration the Temperature Boundary was explored:
Higher Temperatures
- At higher temperatures, molecules or ions within the crystallizing substance typically have more kinetic energy, allowing them to move more freely and rearrange themselves more easily.
- This increased mobility can lead to smoother, rounder crystal shapes because molecules have a greater ability to fill in gaps and form bonds with neighboring particles in a more isotropic manner.
- Crystals grown at higher temperatures may exhibit more rounded edges and smoother surfaces due to the greater mobility of molecules and ions during crystal growth.
Lower Temperatures
- At lower temperatures, molecular mobility decreases, and particles are more likely to remain fixed in position.
- This reduced mobility can result in slower crystal growth and the formation of sharper, more angular crystal shapes.
- Crystals grown at lower temperatures may exhibit sharper edges and more defined facets due to the limited ability of particles to rearrange and fill in gaps during growth.

